The laboratory of living matter and biophysics mainly focuses on theoretical modeling and simulations of soft/active matter and cellular/biological systems. We use different levels of computational models ranging from detailed atomistic models to coarse-grained representations and continuum elastic models to investigate both equilibrium and non-equilibrium dynamic behaviour of soft and active biological and living systems such as cells, cellular organelles, proteins, biomembranes and vesicles, viruses and polymer. To this end, we apply different simulation techniques including Molecular Dynamics simulations, Monte Carlo simulations, and Brownian Dynamics simulations either via in-house developed computer codes using programming languages or through molecular simulations packages such as LAMMPS and GROMACS. Our particular focus is on simulating protein-induced cellular and biological processes such as autophagy, cell migration, endocytosis and cellular uptake of nanoparticles, membrane scission and tubulation, as well as membrane structures and remodeling in cellular organelles and self-assembly of nanostructures on membranes for synthetic biology applications. Some of our recent works follow.

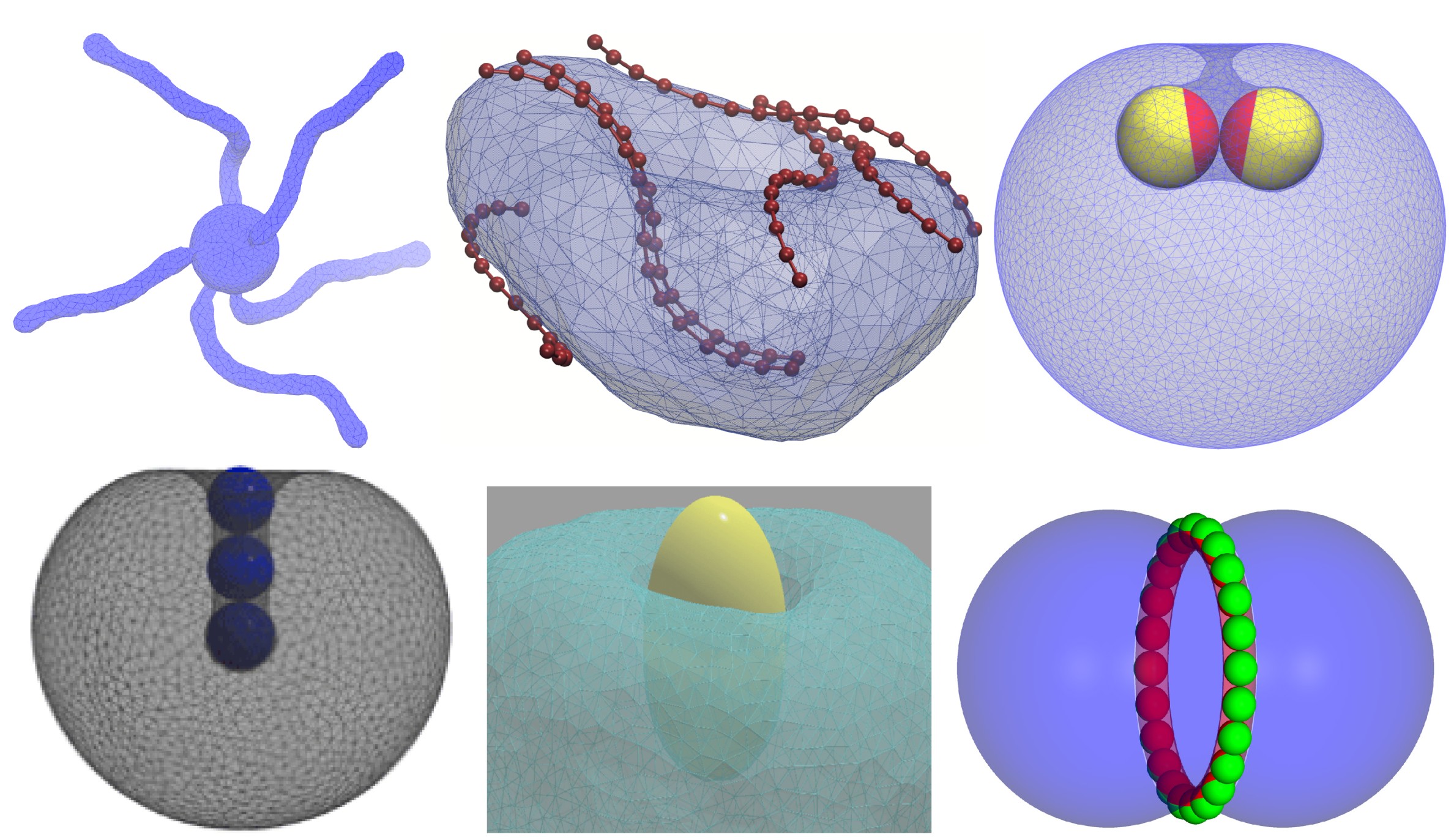
Membrane-mediated interactions between disk-like inclusions
Self-assembly of membrane inclusions plays a key role in biological processes such as cellular signalling and trafficking. How membrane curvature modulates interactions between flat disk-like inclusions, adsorbed on vesicles, remains unknown. We use Monte Carlo simulations of a triangulated vesicle with simulated annealing to explore curvature-mediated interactions between disk-like rigid inclusions, induced by membrane elastic energy. We distinguish two distinct short and long-range curvature-mediated interactions for disk distances below and above the vesicle diameter. Beyond a certain size of disk-like inclusions, we find a transition from neutral to attractive short- range forces. We also demonstrated, in an invited paper to Frontiers in Physics 2022 consistent with experiments, we also show that upon deflating vesicles, previously-attracted disks experience repulsive interactions.

Vesicle compartmentation by phase-separated liquid droplets
Aqueous nanodroplets interact with and shape nanovesicles. We used coarse-grained molecular simulations to explore the architecture of compartmentalized nanovesicles by phase-separated aqueous nanodroplets, and their morphological evolution under osmotic deflation. We showed that phase separation of a biphasic liquid mixture can form both stable two-compartment and meta-stable multi-compartment nanovesicles. We identify morphological transitions of stable two-compartment nanovesicles between tube, sheet and cup morphologies, characterized by membrane asymmetry and phase-separation propensity between the aqueous phases. Our results appeared in RSC Advances 2022, show that aqueous nanodroplets can form novel membrane nanostructures, crucial for cellular processes and forming cellular organelles on the nanoscale.
Stable and unstable compartmentalized nanovesicles

Vesicle constriction by particle rings and clusters of curved proteins
Membrane constriction and scission by proteins and nano structures are crucial to many processes in cellular and synthetic biology. We reported mechanical constriction of vesicles by rings of adsorbed Janus nanoparticles that mimic contractile proteins, and by aggregates of curved crescents that mimic scaffold proteins. We demonstrate that vesicle constriction by crescent aggregates strongly depends on the crescent curvature. Our results appeared in Nanotechnology 2019 offer promising perspectives for designing membrane-constricting nano structures such as nanoparticle aggregates and clusters of synthetic curved proteins such as DNA origami scaffolds with applications in synthetic biology.
Vesicle constriction by curved and ring proteins

Vesicle curvature determines interactions between Janus particles
Besides direct particle–particle interactions, nanoparticles adsorbed to biomembranes experience indirect interactions that are mediated by the membrane curvature arising from particle adsorption. In this Letter, we showed that the curvature-mediated interactions of adsorbed Janus particles depend on the initial curvature of the membrane prior to adsorption, that is, on whether the membrane initially bulges toward or away from the particles in our simulations. The curvature-mediated interaction can be strongly attractive for Janus particles adsorbed to the outside of a membrane vesicle while it is repulsive for inside particles. We find that the area fraction of the adhesive Janus particle surface is an important control parameter for the curvature-mediated interaction, besides the initial membrane curvature as shown in Nano Letters 2018.
Curvature-mediated interactions between Janus particles
Formation and stability of membrane tubules
According to membrane elastic theory, the tubular endoplasmic reticulum (ER), with its high area-to-volume ratio, appears to be particularly unstable. In Monte Carlo simulations of a fluid–elastic membrane model subject to thermal fluctuations, we found that a steady increase in the area-to-volume ratio readily induces tubular structures. Once formed, a high energy barrier separates tubules from the thermodynamically favored sheet-like membrane structures. Remarkably, this barrier persists even at large area-to-volume ratios, protecting tubules against shape transformations despite enormous driving forces toward sheet-like structures. Volume reduction by osmotic regulation and membrane area growth by lipid production and by fusion of small vesicles emerge as powerful factors in the induction and stabilization of tubular membrane structures as shown in ACS Nano 2017.
Membrane tubulation by reducing vesicle volume
Forming the autophgosome in cellular autophagy, (a cellular process that won Nobel prize 2016)
Autophagy is a physiological process for the recycling and degradation of cellular materials. Forming the autophagosome from the phagophore, a cup-shaped double-membrane vesicle, is a critical step in autophagy. The origin of the cup shape of the phagophore is poorly understood. Here, we simulated membrane remodeling processes in the presence and absence of membrane associated Atg17. Our finding approved the scaffolding role of Atg17 complexes in forming the phagophore. We confirmed the critical role of Atg17-membrane interactions experimentally by showing that mutations of putative membrane interaction sites result in reduction or loss of autophagic activity in yeast. Our results appeared in PLOS computational Biology 2016.
Scaffolding post-fusion structure of 3 vesicles into phagophore by 6 Atg crescents

Orientational change of elongated nanoparticle during internalization
Wrapping and internalization of nanoparticles by biomembranes play a critical role in drug delivery applications and nanomedicine. Here we studied the wrapping process of a vesicle membrane around spherical and ellipsoidal nanoparticles via minimization of the bending and adhesion energies. We reported two distinct regimes of spreading and internalization separated by an energy barrier and related the success or failure of the internalization to the particle shape and wrapping orientation. We predicted more difficult internalization for ellipsoidal particles with higher aspect ratios. Wrapping of ellipsoidal particles is associated with an orientational change of the particle. While the spreading starts on the flat side of the ellipsoidal particle, the particle changes its orientation during wrapping and the internalization is achieved in the tip orientation of both prolate and oblate ellipsoidal particles. More details can be found in our paper on the cover of Soft Matter 2013.
Cellular uptake of elongated nanoparticles by vesicle membrane

Aggregation and tubulation of spherical nanoparticles on vesicles
How nanoparticles interact with biomembranes is central for understanding their bioactivity. In this Letter, we reported novel tubular membrane structures induced by adsorbed spherical nanoparticles, which we obtained from energy minimization. The membrane tubules enclose linear aggregates of particles and protrude into the vesicles. The high stability of the particle-filled tubules implies strongly attractive, membrane-mediated interactions between the particles. The tubular structures may provide a new route to encapsulate nanoparticles reversibly in vesicles. Our results appeared in a suggested paper by editor in Physical Review Letters 2012.
(a) membrane-mediated attracted nanopartciles on a vesicle (b) attracted particles in membrane tubule